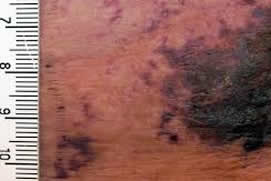
Laboratoris Sanifit, a biotechnology company which began within the University of the Balearic Islands (UIB), has received authorisation from the UK’s Medicines and Healthcare Products Regulatory Agency in order to begin Phase I clinical trials of a drug to combat calciphylaxis, a rare and fatal disease.
This disease results in massive vascular calcification of small peripheral blood vessels in some diabetic patients, and currently there is no treatment for it. The mortality rate for this illness, which affects between 1% and 4% of dialysis patients, is very high at over 80%. Once a patient is diagnosed with the disease average life expectancy is only one year.
This drug now entering Phase I trials has been successfully tested on mice. Called SNF472, the experimental compound has demonstrated an excellent safety-efficacy profile in different animal models. Sanifit has already received orphan drug designation for SNF472 from the EMA (European Medicines Agency) and the FDA (Food and Drug Administration) in the US.
The company licensed its first product in 2013 for treatment of dental calcification (plaque). This product is added to toothpastes and mouthwashes, and is based on the same scientific principles for fighting calcification.